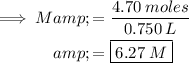SOLUTION:
The molarity of a solution is defined as the number of moles of solute per liter of solution.
We first need to convert the volume of the solution from milliliters to liters:
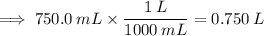
Now we can calculate the molarity (M) using the formula:
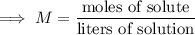
Substituting the given values: