SOLUTION:
The balanced chemical equation for the reaction between nitrogen gas (
 ) and hydrogen gas (
) and hydrogen gas (
 ) is:
) is:
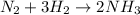
According to the stoichiometry of the reaction, 1 mole of
 reacts with 3 moles of
reacts with 3 moles of
 to produce 2 moles of
to produce 2 moles of
 .
.
Therefore, to determine how many moles of
 are needed to react with 7.5 moles of
are needed to react with 7.5 moles of
 , we need to use the mole ratio between
, we need to use the mole ratio between
 and
and
 :
:
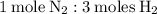
We can use this ratio to set up a proportion:
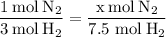
Solving for x, we get:
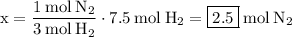
 2.5 moles of
2.5 moles of
 are needed to react with 7.5 moles of
are needed to react with 7.5 moles of
 .
.
