The Arrhenius acids among the given options are
 (c) and
(c) and
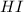 (d).
(d).
Arrhenius acids are substances that, when dissolved in water, increase the concentration of hydronium ions
 . Let's evaluate the options:
. Let's evaluate the options:
a.
 (water) - Water itself can act as both an acid and a base, but it does not significantly increase
(water) - Water itself can act as both an acid and a base, but it does not significantly increase
 concentration when pure. It is considered amphoteric, not a typical Arrhenius acid.
concentration when pure. It is considered amphoteric, not a typical Arrhenius acid.
b.
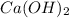 (calcium hydroxide) - This is a base because it dissociates in water to produce hydroxide ions
(calcium hydroxide) - This is a base because it dissociates in water to produce hydroxide ions
 , not
, not
 . It is not an Arrhenius acid.
. It is not an Arrhenius acid.
c.
 (phosphorous acid) - This is an acid as it can donate a proton
(phosphorous acid) - This is an acid as it can donate a proton
 in water. It is an Arrhenius acid.
in water. It is an Arrhenius acid.
d.
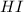 (hydroiodic acid) - This is an acid as it readily dissociates in water to produce
(hydroiodic acid) - This is an acid as it readily dissociates in water to produce
 and
and
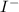 . It is an Arrhenius acid.
. It is an Arrhenius acid.
Therefore, the Arrhenius acids among the given options are
 (c) and
(c) and
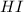 (d).
(d).