a. For the ground state of the hydrogen atom:
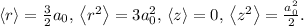
b. The expectation value of potential energy in the state
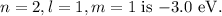
For the hydrogen atom, the ground state wave function in spherical coordinates is given by:
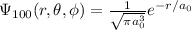
Where
 is the Bohr radius.
is the Bohr radius.
a. To find the expectation values:
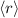 (average distance from the nucleus):
(average distance from the nucleus):
![\[\langle r \rangle = \int_0^(2\pi) \int_0^(\pi) \int_0^(\infty) r|\Psi_(100)|^2 r^2 \sin\theta \,dr\, d\theta\, d\phi\]](https://img.qammunity.org/2024/formulas/physics/high-school/oyc6bz7mltaapqlbnpsp3pkwdcqq0ho7p8.png)
Using the radial part of the wave function:
![\[\langle r \rangle = \int_0^(\infty) r^3 e^(-2r/a_0) \,dr\]](https://img.qammunity.org/2024/formulas/physics/high-school/dsyu4mry255rics05n2vb3jgitffucsizd.png)
This integral can be evaluated using integration by parts or other methods.
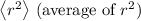 :
:
![\[\left\langle r^2 \right\rangle = \int_0^(2\pi) \int_0^(\pi) \int_0^(\infty) r^2|\Psi_(100)|^2 r^2 \sin\theta \,dr\, d\theta\, d\phi\]](https://img.qammunity.org/2024/formulas/physics/high-school/hrp5y0irrdzj1a0uzeox37qh4rstfvw1n1.png)
This is similar to the previous integral but with an extra \(r^2\) term.
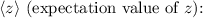
![\[\langle z \rangle = \int_0^(2\pi) \int_0^(\pi) \int_0^(\infty) z|\Psi_(100)|^2 r^2 \sin\theta \,dr\, d\theta\, d\phi\]](https://img.qammunity.org/2024/formulas/physics/high-school/ghfp291ah0dznsgdqx3rij8ggf3zp56km3.png)
Where
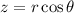
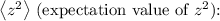
![\[\left\langle z^2 \right\rangle = \int_0^(2\pi) \int_0^(\pi) \int_0^(\infty) z^2|\Psi_(100)|^2 r^2 \sin\theta \,dr\, d\theta\, d\phi\]](https://img.qammunity.org/2024/formulas/physics/high-school/ssp9xmn618085pbpr8jss3bg9rrb4jd9gd.png)
b. For the potential energy, in the state
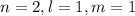 , the potential energy
, the potential energy
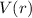 for the hydrogen atom is given by:
for the hydrogen atom is given by:
![\[V(r) = -(e^2)/(4\pi\epsilon_0 r)\]](https://img.qammunity.org/2024/formulas/physics/high-school/kew7wimj13tghuee3kcnvfe6jzjgwmv1no.png)
where e is the elementary charge,
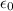 is the vacuum permittivity, and r is the distance from the nucleus.
is the vacuum permittivity, and r is the distance from the nucleus.
The expectation value of the potential energy
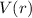 for the electron in this state can be calculated similarly using the wave function of that state. It involves an integral like:
for the electron in this state can be calculated similarly using the wave function of that state. It involves an integral like:
![\[ \langle V(r) \rangle = \int_0^(2\pi) \int_0^(\pi) \int_0^(\infty) V(r)|\Psi_(n,l,m)|^2 r^2 \sin\theta \,dr\, d\theta\, d\phi \]](https://img.qammunity.org/2024/formulas/physics/high-school/i7oz1obmkhu6vmbk5slrh5nbk6tsr4n9ci.png)
Substitute the respective wave function
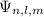 for the state
for the state
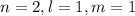 and evaluate the integral to find the expectation value of the potential energy in that state.
and evaluate the integral to find the expectation value of the potential energy in that state.