To calculate the molar solubility of XF₂ in a 0.199 M X(NO₃)₂ solution, we need to use the solubility product constant (Ksp) and the stoichiometry of the balanced equation.
The balanced equation for the dissolution of XF₂ is:
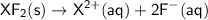
According to the equation, 1 mole of XF₂ produces 1 mole of X²⁺ ions and 2 moles of F⁻ ions.
Let's assume the molar solubility of XF₂ is represented by "x" (in mol/L).
The equilibrium expression for the solubility product (Ksp) is:
![\displaystyle\sf Ksp = [X^(2+)] * [F^(-)]^2](https://img.qammunity.org/2024/formulas/chemistry/high-school/nojs58zi927a0kz9dxr3510vma98rgjcy8.png)
Given that Ksp = 5.3 x 10⁻⁶ and the concentration of X(NO₃)₂ is 0.199 M, we can set up the following equation:
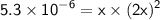
Solving for "x," we find:
![\displaystyle\sf x \approx \sqrt[3]{(5.3 * 10^(-6))/(4)}](https://img.qammunity.org/2024/formulas/chemistry/high-school/m37872pwzisprci1pjkp9po7ep6wdql7sz.png)
Calculating the result, we get:

Therefore, the molar solubility of XF₂ in the 0.199 M X(NO₃)₂ solution is approximately 0.0197 mol/L.