Answer:
0.164 M
Step-by-step explanation:
Molarity is defined as moles of solute per liter of solution. In order to arrive at the desired units, dimensional analysis must be performed.
To find moles of sodium:
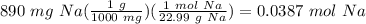
To find liters of solution:
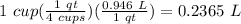
The ratio of sodium to solution can then be written as
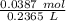 . This can be simplified by dividing the numbers,
. This can be simplified by dividing the numbers,
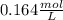 or 0.164 M.
or 0.164 M.