Final answer:
The absolute molar entropy (S) at a low temperature (T) for most substances can be calculated by integrating the heat capacity (Cp) over temperature, using Cp = a
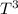 here 'a' is a constant).
here 'a' is a constant).
Step-by-step explanation:
In the context of low temperatures, it is observed that the heat capacity (Cp) of most substances is directly proportional to the cube of the temperature (T3). According to the third law of thermodynamics, the absolute molar entropy (S) can be determined by integrating the heat capacity over temperature from absolute zero to the temperature of interest. Specifically, S is found by the area under the Cp/T vs T curve from 0 K to a given temperature T.
To write an expression for S at a low temperature T, in terms of Cp, the relationship Cp =
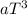 'a' is a constant) can be used. Therefore, the expression for absolute molar entropy, assuming the validity of the Debye approximation, is given by the integral:
'a' is a constant) can be used. Therefore, the expression for absolute molar entropy, assuming the validity of the Debye approximation, is given by the integral:
S = ∫(0, T) (aT3) / T dT = a ∫(0, T) T2 dT = aT3 / 3