The reagent combination that can be used to convert 1-pentyne into a ketone is
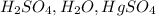 . Option 4.
. Option 4.
The conversion of 1-pentyne into a ketone involves multiple steps. Let's evaluate the provided reagents:
1.
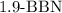 - This is a boron-based reducing agent and is not typically used to convert alkynes into ketones. It is more commonly employed for reduction reactions.
- This is a boron-based reducing agent and is not typically used to convert alkynes into ketones. It is more commonly employed for reduction reactions.
2.
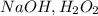 - This is a reagent combination that can be used for oxidative cleavage of alkynes. In the case of 1-pentyne, it would result in the formation of carboxylic acids, not a ketone.
- This is a reagent combination that can be used for oxidative cleavage of alkynes. In the case of 1-pentyne, it would result in the formation of carboxylic acids, not a ketone.
3.
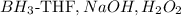 - This combination involves hydroboration-oxidation. It is known to convert alkynes into aldehydes, not ketones.
- This combination involves hydroboration-oxidation. It is known to convert alkynes into aldehydes, not ketones.
4.
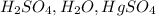 - This combination is commonly used for the hydration of alkynes, resulting in the formation of ketones.
- This combination is commonly used for the hydration of alkynes, resulting in the formation of ketones.
5.
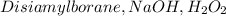 - Similar to option 3, this combination involves hydroboration-oxidation, leading to the formation of aldehydes, not ketones.
- Similar to option 3, this combination involves hydroboration-oxidation, leading to the formation of aldehydes, not ketones.
Therefore, the reagent combination that can be used to convert 1-pentyne into a ketone is
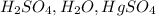 (option 4).
(option 4).