2 mL of the reconstituted solution would contain the ordered dosage of 200 mg of acyclovir for the patient with shingles.
How to determine the quantity that would be contained the ordered dosage?
To determine how many mL would contain the ordered dosage, we can use the formula below to know the concentration of acyclovir.
![\[\text{Concentration of acyclovir} = \frac{\text{Amount of acyclovir}}{\text{Volume of solution}}\]](https://img.qammunity.org/2024/formulas/physics/high-school/fngpabyblzpl78z31v72q22r5cojassjeg.png)
Where;
Acyclovir amount = 0.5 g
Volume of solution = 5 mL
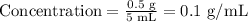
Let us proceed to find the volume needed to contain the ordered dosage of 200 mg of acyclovir (which is 0.2 g):
We can use the formula:
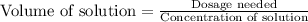
Given:
Dosage needed = 200 mg = 0.2 g
Concentration of solution = 0.1 g/mL
Therefore,
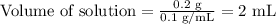
Hence, 2 mL of the reconstituted solution would contain the ordered dosage of 200 mg of acyclovir for the patient with shingles.