Both the rock and the water will experience an approximate temperature rise of
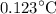 .
.
To find the rise in temperature of the rock and water, we can use the principle of conservation of energy. The potential energy lost by the falling rock will be converted into the thermal energy gained by the rock and water.
The potential energy lost by the rock as it falls can be calculated using the formula:
![\[ \text{Potential energy} = \text{mass} * \text{gravity} * \text{height} \]](https://img.qammunity.org/2024/formulas/physics/high-school/pjs3rkxgyn154xqxq1r106q5lmfqorflpl.png)
The formula for potential energy is
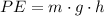 , where:
, where:
-
 is the mass of the rock (0.298 kg)
is the mass of the rock (0.298 kg) -
 is the acceleration due to gravity (9.81 m/s²)
is the acceleration due to gravity (9.81 m/s²) -
 is the height (23.1 m)
is the height (23.1 m)
Let's calculate the potential energy lost by the rock:
![\[ PE = 0.298 \, \text{kg} * 9.81 \, \text{m/s}^2 * 23.1 \, \text{m} \]](https://img.qammunity.org/2024/formulas/physics/high-school/tzk04nghbtngc4ofmwhodyrul65lwseh0z.png)
![\[ PE \approx 68.817 \, \text{J} \]](https://img.qammunity.org/2024/formulas/physics/high-school/87og5bzo3vg6y0xt59d5nxpongiuzejpys.png)
This potential energy will be converted into the thermal energy gained by the rock and the water.
The thermal energy gained by the rock can be calculated using the specific heat formula:
![\[ \text{Thermal energy} = \text{mass} * \text{specific heat} * \Delta \text{temperature} \]](https://img.qammunity.org/2024/formulas/physics/high-school/xzi7jamewil1g401isu1l3hmi2ln6xcgnb.png)
Let (T) be the rise in temperature for both the rock and the water. The thermal energy gained by the rock and water will be:
For the rock:
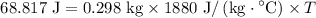
Solving for (T) (the rise in temperature of the rock):
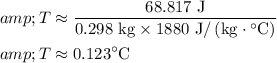
Therefore, The answer is
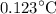 .
.