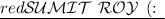To find the freezing point of the solution, we can use the formula for freezing-point depression:
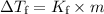
where:
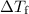 is the freezing-point depression,
is the freezing-point depression,
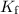 is the freezing-point depression constant, and
is the freezing-point depression constant, and
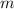 is the molality of the solution.
is the molality of the solution.
First, we need to calculate the molality of the solution. The molality is defined as the number of moles of solute per kilogram of solvent. In this case, the solvent is paradichlorobenzene.
Step 1: Calculate the number of moles of benzene (solute):
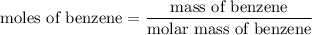
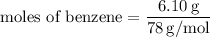
Step 2: Calculate the mass of paradichlorobenzene (solvent):
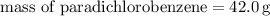
Step 3: Calculate the molality of the solution:
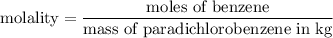
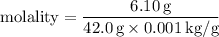
Now that we have the molality, we can calculate the freezing-point depression.
Step 4: Calculate the freezing-point depression:
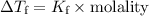
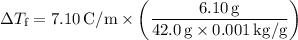
Finally, we can calculate the freezing point of the solution.
Step 5: Calculate the freezing point:
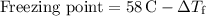
Simplify and compute the values to find the freezing point of the solution.
♥️