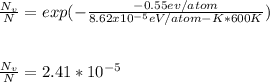All materials have some imperfections and, some of these can improve or harm material properties.
The material imperfections can be classified as point, surface, or volumetric defects. The vacancy, interstitial atom, small/large substitutional atom, Frenkel defect, and Schottky defect are classified as point defects.
Some point material defects are influenced by temperature, for example: the vacancies. For finding the number of vacations is necessary to apply the equation:
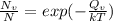 , where:
, where:
Nv= the number of vacancies/cm³;
n=the number of atoms/cm³;
Qv=the energy required to produce one mole of vacancies, in cal/mol or Joules/mol;
T =the temperature in degrees Kelvin.
k= 8.62 x 10-5 eV/atom-K
The question gives:
T =600K
Material = gold, then its Qv=0.55 eV/atom.
Nv= the number of vacancies/cm³;
T =600K.
Applying the previous equation presented, you have: