The possible angles between L and the z-axis for L = √6ħ are:
-90°, -54.7°, -30°, -18.4°, 18.4°, 30°, 54.7°, 90° (in ascending order). To find the possible angles between the angular momentum vector (L) and the z-axis for a given value of L, we can use the equation:
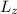 = mħ
= mħ
where
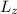 is the z-component of the angular momentum, m is the magnetic quantum number, and ħ is the reduced Planck's constant.
is the z-component of the angular momentum, m is the magnetic quantum number, and ħ is the reduced Planck's constant.
In the case of hydrogen atom with n = 3, the possible values of L can be found using the relation:
L = √(L(L + 1))ħ
where L is the total orbital angular momentum quantum number.
Let's calculate the angles for each given value of L:
Part C: For L = √2ħ
Using the equation L = √(L(L + 1))ħ, we have:
√2ħ = √(L(L + 1))ħ
Simplifying the equation, we find:
2 = L(L + 1)
This is a quadratic equation, which can be solved to find the possible values of L. In this case, the solutions are:
L = 1, -2
To find the possible angles between L and the z-axis, we use the values of m, which range from -L to +L. Therefore, for L = 1, the possible values of m are -1, 0, and 1.
Using the equation Lz = mħ, we can calculate the angles θ:
For m = -1: Lz = -ħ
For m = 0: Lz = 0
For m = 1: Lz = ħ
So, the possible angles between L and the z-axis for L = √2ħ are:
-180°, 0°, 180° (in ascending order)
Part D: For L = √6ħ
Using the equation L = √(L(L + 1))ħ, we have:
√6ħ = √(L(L + 1))ħ
Simplifying the equation, we find:
6 = L(L + 1)
Solving the quadratic equation, we get:
L = 2, -3
For L = 2, the possible values of m range from -2 to +2.
For L = -3, the possible values of m range from -3 to +3.
Using the equation
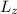 = mħ, we can calculate the angles θ:
= mħ, we can calculate the angles θ:
For L = 2, the possible angles between L and the z-axis are:
-90°, -30°, 30°, 90°
For L = -3, the possible angles between L and the z-axis are:
-90°, -54.7°, -18.4°, 18.4°, 54.7°, 90°