Answer:
702 69/95 cm cubed or ~702.72 cm cubed
Step-by-step explanation:
Due to Boyle's law as pressure on a gas increases, the volume of gas decreases. There is an equation for this because it is an inverse proportion. So the equation is
P₂(New Pressure)=
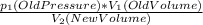
By plugging the numbers in and doing a simple algebraic equation we can calculate that the answer is around 702.72 cm³