The volume of 63.0g of water at a temperature of 84°C is approximately 65.03 mL.
The density of water is defined as mass per unit volume. In this case, the density of water at 84°C is given as 0.969 g/mL.
To calculate the volume of water, we can rearrange the density formula as follows:

Given that the mass of water is 63.0g, we can substitute these values into the formula:
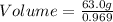 g/mL
g/mL
To ensure consistent units, we need to convert the density to grams per gram (g/g):
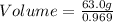 g/g
g/g
Simplifying the calculation:
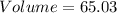 g/g
g/g
Therefore, the volume of 63.0g of water at a temperature of 84°C is approximately 65.03 mL.