Step-by-step explanation:
✰
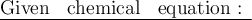
Here , Multiply H₂ by 3 on left hand side and H₃ by 2 on right hand side to equalize hydrogen atoms.
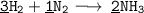
Here , We have 6 hydrogen atoms in both sides. How about Nitrogen atoms? We have 2 Nitrogen atoms in both sides. And yippie !! We got the balanced equation where both the hydrogen and nitrogen atoms are equal.
Hope I helped ! ♡
Have a wonderful day / night ! ツ
#
 !!
!!
▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁