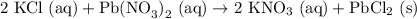Step-by-step explanation:
Part 1:
When a soluble ionic compound dissolves in water, it completely separates into its constituent ions. For example, NaCl completely separates into Na+ and Cl- ions floating around in the water.
The water molecules will surround the ions, but in a very special way. The positive ions (like Na+) will be surrounded by water molecules with their oxygen atom pointing towards the positive ion. The negative ions (like Cl-) will be surrounded by water molecules with their hydrogen atoms pointing towards the negative ion.
(Sketch at the end).
Part 2:
Balanced Chemical Equation
To write the balanced chemical equation for the reaction between Iron (III) chloride (FeCl3) and potassium hydroxide (KOH), we must first think about the chart on each ion. Let's start with FeCl3.
Since we are told that the substance is Iron (III) chloride, we know that the iron atom has a positive +3 charge (the Roman numeral notation tells you what the positive chart on the element is). Since chlorine has seven valence electrons, its ion will have a negative -1 charge. So far, we have:
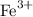 and
and
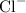
For potassium hydroxide (KOH), we should memorize that the OH ion has a negative -1 charge, while the potassium ion has a positive +1 charge. So, we have:
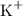 and
and
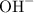
Now, for the main event. When two ionic substances are combined in aqueous solution, the ions will swap places. So, the Fe atom will attach to the OH molecule, and the K atom will attach to the Cl atom.
- Since the Fe has a charge of +3, we will need three OH ions to balance the resulting substance:
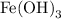
- Since K has a charge of +1, we will only need one Cl ion to balance the resulting substance:
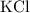
Our first draft of the equation will be:
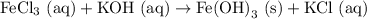
However, this is wrong since the Cl and OH numbers are not balanced! We have 3 OH molecules on the right, so we need a "3" in front of the KOH on the left:
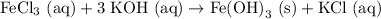
But now, we have 3 K atoms on the left, and only one on the right, so we need a "3" in front of the KCl on the right! Finally! The equation is balanced. Our final balanced chemical equation is:
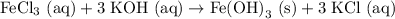
Complete Ionic Equation
To write the complete ionic equation, we break apart all the ionic aqueous substances in the balanced chemical equation we just wrote. It's not always obvious which substances to break apart and which to leave together, but a common memory aid is the "SNAP" ions: sodium (Na), nitrate (NO3), ammonia (NH4), and potassium (K) usually break apart. This gives us our complete ionic equation:
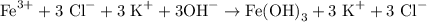
Note that the solid precipitate Fe(OH)3 will NOT break apart.
Net Ionic Equation
To write the net ionic equation, cross out any ions that are the same on both sides. What's left is:
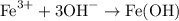
Part 3:
To predict the products of the combinations of reacts, we follow a similar procedure to the "Balanced chemical equation" section above.
Na2CO3 (aq) with Ni(NO3)2 (aq)
Na = +1 charge (memorized)
CO3 = -2 charge (to balance the 2 Na's)
NO3 = -1 charge (memorized)
Ni = +2 charge (to balance the 2 NO3's)
First draft (swapping ions):
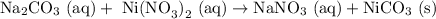
From the first draft, we can already see that the product should be NiCO3 (the other molecule NaNO3 has sodium which will stay in solution).
Balancing with the proper amount of each atom gives us the balanced equation (just needs a "2" on the NaNO3):
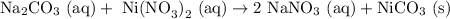
KCl (aq) with Pb(NO3)2 (aq)
K = +1 charge (memorized)
Cl = -1 charge (memorized)
NO3 = -1 charge (memorized)
Pb = +2 charge (to balance the 2 NO3's)
First draft (swapping ions):
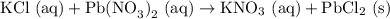
We predict that the product should be PbCl2 (the other molecule has potassium which will stay in solution).
Balancing with the proper amount of each atom gives us the balanced equation: