Answer: The volume of an ideal gas will decrease if the pressure increases.
Step-by-step explanation:
To answer this question, we need to use the Ideal Gas Law:

The "mathematical" way of solving this is to solve the equation for the variable we are interested in. In this case, we want to know about the volume, so get V by itself:
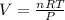
By inspecting the formula, we can see that n, R, and T are in the numerator, while P is in the denominator. Mathematically speaking, if the numerator changes the SAME change will happen to V; if the denominator changes the OPPOSITE change will happen to V.
For example,
- If n increases, the same thing happens to V. V increases.
- If T increases, the same thing happens to V. V increases.
- If n decreases, the same thing happens to V. V decreases.
- If T decreases, the same thing happens to V. V decreases.
But, for the denominator,
- If P increases, the OPPOSITE thing happens to V. V decreases.
- If P decreases, the OPPOSITE thing happens to V. V increases.
So, since the questions says that the pressure changes from 15 atm to 30 atm (an INCREASE), the volume will do the OPPOSITE thing and decrease.
General Patterns:
The following are general patterns for the Ideal Gas Law:
- P does the SAME thing as n and T.
- P does the OPPOSITE thing as V.
- V does the SAME thing as n and T.
- V does the OPPOSITE thing as P.
- n does the SAME thing as P and V.
- n does the OPPOSITE thing as T.
- T does the SAME thing as P and V.
- T does the OPPOSITE thing as n
- R is a constant so it never changes.