Answer: The molarity of the acid is 1.5 M.
Step-by-step explanation:
We can use the equation:
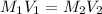
where M represents the molarity of the solution, and V represents the volume.
Then, plugging in our information, we have:
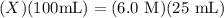
Solving for X, by dividing by 100, gives us:
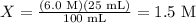
So, the molarity of the unknown acid is 1.5 M.