Answer: The equilibrium constant of its reverse reaction is 0.206.
Step-by-step explanation:
For a given reaction if, the forward reaction has an equilibrium constant of
 , then the reverse reaction has an equilibrium constant of
, then the reverse reaction has an equilibrium constant of
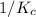 .
.
In the question,
 . Therefore, the reverse reaction has an equilibrium constant of
. Therefore, the reverse reaction has an equilibrium constant of
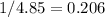 .
.
More Details:
You can show yourself that this rule works, by the following process.
Suppose you have a chemical reaction given by:
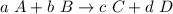
Then, the equilibrium constant for this reaction is given by the formula:
![K_c=([C]^c[D]^d)/([A]^a[B]^b)](https://img.qammunity.org/2024/formulas/chemistry/college/dzu9bdblngzaaf8smkhy7z9vj0evxo9fby.png)
On the other hand, the reverse reaction is given by:
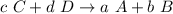
Then, the reverse reaction's equilibrium constant is:
![K_r=([A]^a[B]^b)/([C]^c[D]^d)](https://img.qammunity.org/2024/formulas/chemistry/college/vtu04rgrfmmw28mpv3eryy25rcb6zp85vu.png)
But this is the same as the original constant, except it's flipped over! Mathematically, speaking:
![K_r = ([A]^a[B]^b)/([C]^c[D]^d) = 1 / ([C]^c[D]^d)/([A]^a[B]^b) = 1 / K_c = 1 / K_c](https://img.qammunity.org/2024/formulas/chemistry/college/n48grvwi3vdy6w7oao7bp0i2dt1vnh25hz.png)