Answer: X = 1
Step-by-step explanation:
We need to determine the number of water molecules in a hydrate. The chemical reaction we write is:
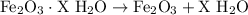
From the given information, we started with 26.7 g of hydrated compound, but after heating, were only left with 24.0 g of mass. Since mass is conserved, the missing 2.7 g must belong to the water vapor that evaporated from the compound.
Determining Moles
2.7 g of H2O x 1 mol H2O / (18.016 g H2O) = 0.15 mol H2O
24.0 g of Fe2O3 x 1 mol Fe2O3 / (159.70 g Fe2O3) = 0.15 mol Fe2O3
This means that the number of moles of H2O : number of moles of Fe2O3 is exactly 1 : 1. In other words, the balanced equation must be:
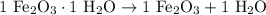
So, the value of X = 1.