Answer:
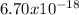 g
g
Step-by-step explanation:
The molar mass of naturally occurring neon (Ne) is approximately 20.18 grams/mol.
To calculate the mass of
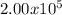 atoms of neon, we can use the following steps:
atoms of neon, we can use the following steps:
Determine the number of moles: Divide the number of atoms by Avogadro's number (
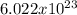 Atoms/MOL).
Atoms/MOL).
Number of moles = (
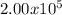 atoms) / (
atoms) / (
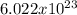 atoms/mol)
atoms/mol)
Calculate the mass: Multiply the number of moles by the molar mass of neon.
Mass = (Number of moles) × (Molar mass of neon)
Using the above steps, we can calculate the mass as follows:
Number of moles = (
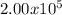 atoms) / (
atoms) / (
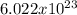 atoms/mol)
atoms/mol)
≈
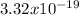 moles
moles
Mass = (
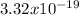 moles) × (20.18 grams/mol)
moles) × (20.18 grams/mol)
≈
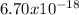 grams
grams
Therefore, the mass of
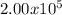 Atoms of naturally occurring neon is approximately
Atoms of naturally occurring neon is approximately
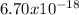 Grams.
Grams.