Answer:
0.39 M
Step-by-step explanation:
Using the formula: M = moles of solute / liters of solution
Before that, convert 2.3 grams of NaCl to moles:
2.3 grams ×
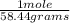 = 0.03935660507 moles
= 0.03935660507 moles
Convert given mL to L:
100 mL ×
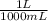 = 0.1 L
= 0.1 L
Calculate the molarity of the solution:
M = 0.03935660507 moles / 0.1 = 0.39 M