Answer:
0.005 moles
Step-by-step explanation:
To find the number of moles of HCl in a concentrated solution, we can use the formula for molarity:
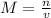 , where M = molarity, n = moles of solute, and v = volume of solution in liters.
, where M = molarity, n = moles of solute, and v = volume of solution in liters.
In order to plug the known values in the formula, we must first convert 10 mL to L. There are 1000 mL in a liter, so we divide 10 by 1000 to get 0.01 L of solution.
We can then rearrange the molarity formula to solve for n and then plug in the known values:
n = M × v
n = (0.5 mol/L) × (0.01 L)
n = 0.005 mol
Therefore, there are 0.005 moles of HCl in the solution.