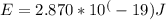Final answer:
The energy of the emitted photon is approximately 2.870 x 10^(-19) joules.
Step-by-step explanation:
The energy of a photon can be calculated using the formula:
E = hc/λ
where E is the energy, h is Planck's constant, c is the speed of light
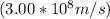 , and λ is the wavelength.
, and λ is the wavelength.
In this case, the wavelength is given as 435.8 nm. To convert it to meters, we divide by 10^9:
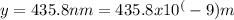
Plugging these values into the formula, we get:
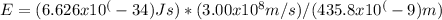
Simplifying the expression gives us the energy of the emitted photon: