The final volume of the balloon, when completely cooled in the refrigerator, will be approximately 0.32 liters.
To solve this problem, we need to use Charles's law, which states that, at constant pressure, the volume of a sample of gas is directly proportional to its temperature.
The law can be expressed mathematically as:
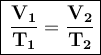
Where:
- V₁ is the initial volume
- T₁ is the initial temperature
- V₂ is the final volume
- T₂ is the final temperature
Now we obtain the data to continue solving:
- V₁ = 0.5 L
- T₁ = 220 °C
- V₂ = ?
- T₂ = 40 °C
Now, we will convert the temperatures to Kelvin:


Now we solve for V₂:
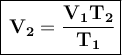
Where:
- V₁ is the initial volume
- T₁ is the initial temperature
- V₂ is the final volume
- T₂ is the final temperature
Now, we substitute the values in the formula:
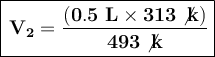

The final volume of the balloon, when completely cooled in the refrigerator, will be approximately 0.32 liters.