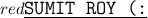The most reactive nonmetals are the elements in Groups 6 and 7, which corresponds to option B. These groups include elements such as oxygen (O), sulfur (S), fluorine (F), chlorine (Cl), and others. Nonmetals in these groups tend to have high electronegativity values and a strong tendency to gain electrons in chemical reactions, making them highly reactive.
♥️