Answer:
The new pressure will be 0.494 atm.
Step-by-step explanation:
We can use the ideal gas law to solve this problem:
PV = nRT
where:
- P is the pressure
- V is the volume
- n is the number of moles of gas
- R is the ideal gas constant
- T is the temperature
We know that the initial pressure is 0.988 atm, the initial volume is 1 L, and the temperature is constant.
We also know that the final volume is 2 L.
We can solve for the final pressure as follows:
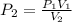
substituting value
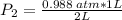
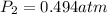
Therefore, the new pressure will be 0.494 atm.