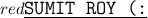The correct answer is B. The solute bonds to the solvent, forming new compounds.
When a material is dissolved in a solvent, the solute particles become dispersed and surrounded by solvent molecules. This process typically involves the solute molecules or ions breaking apart and interacting with the solvent molecules through various intermolecular forces such as hydrogen bonding, dipole-dipole interactions, or ion-dipole interactions.
As a result, new compounds or species are formed in the solution, where the solute particles are now incorporated within the solvent. This allows for the homogeneous mixing of the solute and solvent at the molecular or ionic level, resulting in a uniform distribution of particles throughout the solvent.
♥️