Answer: 12.5%
Explanation:
Formula for Half Life
N = N₀
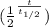
N = number of isotopes after
N₀ = Initial Isotopes
t = number of years
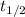 = half life years
= half life years
Given
t=6000
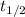 = 2000
= 2000
Percent is part out of the whole = remaining/starting amoung
Percent fraction =
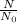
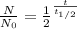
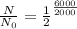
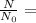 .125 =12.5%
.125 =12.5%