Answer: 0.983 moles of carbon dioxide are present
Step-by-step explanation:
To determine the number of moles of carbon dioxide (CO₂) present when there are 11.8 x 10²³ atoms of oxygen, we need to consider the molecular formula of carbon dioxide.
The molecular formula of carbon dioxide (CO₂) indicates that there is one carbon atom and two oxygen atoms in each molecule of carbon dioxide.
Given that there are 11.8 x 10²³ atoms of oxygen, we can divide this number by 2 to determine the number of carbon dioxide molecules present, since each molecule of carbon dioxide contains two oxygen atoms.
Number of carbon dioxide molecules =
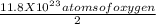
Next, we can use Avogadro's number, which states that there are approximately 6.022 x 10²³molecules per mole, to convert the number of carbon dioxide molecules to moles.
Number of moles of carbon dioxide = (11.8 x 10²³ atoms of oxygen / 2) / (6.022 x 10^23 molecules per mole)
Evaluating this expression, we get:
Number of moles of carbon dioxide = 0.983 moles