Answer:
- i. Write the reaction sequence of the conversion of A to D.
Dehydration reaction:
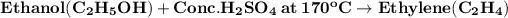
Hydrogenation reaction:

Chlorination reaction:
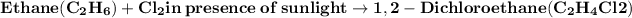
Wurtz reaction:
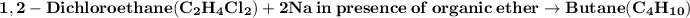
- ii. Write the IUPAC name of C and D.
 IUPAC name=ethane
IUPAC name=ethane
D=
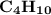 IUPAC name=Butane
IUPAC name=Butane
- iii. How can you prove chemically the compound A is unsaturated?
To prove chemically that compound A is unsaturated, you can add bromine water to it. If the bromine water decolorizes, then it means that compound A is unsaturated.
- iv. Write the name of the reaction for the conversion of compound C to D.
The name of the reaction for the conversion of compound C to D is the Wurtz reaction.
Note:
Detailed explanations of the reactions:
- The reaction of ethanol with conc. H2SO4 at 170°C is a dehydration reaction. This reaction removes water from ethanol to produce ethylene.
- The reaction of ethylene with hydrogen in the presence of a Pt catalyst is a hydrogenation reaction. This reaction adds hydrogen to ethylene to produce ethane.
- The reaction of ethane with chlorine in the presence of sunlight is a chlorination reaction. This reaction adds chlorine to ethane to produce 1,2-dichloroethane.
- The reaction of 1,2-dichloroethane with sodium in the presence of organic ether is a Wurtz reaction. This reaction removes chlorine from 1,2-dichloroethane to produce butane.