Answer:

Step-by-step explanation:
Given data:
Pressure 1 =
 = 0.860 atm
= 0.860 atm
Volume 1 =
 = 11.2 L
= 11.2 L
Volume 2 =
 = 15.2 L
= 15.2 L
Required:
Pressure 2 =
 = ?
= ?
Formula:
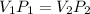 (Boyle's Law)
(Boyle's Law)
Solution:
Put the given data in the above formula.
(11.2)(0.860) = (15.2)(P₂)
9.632 = (15.2)(P₂)
- Divide both sides by 15.2
9.632 / 15.2 = P₂
P₂ ≈ 0.63 atm
![\rule[225]{225}{2}](https://img.qammunity.org/2024/formulas/mathematics/high-school/yk3l6bxs46equldxx0z8qla0zuvm8l328e.png)