The number of mole of H₂ that are required to react with 3.2 moles Cl₂ is 3.2 moles
How to calculate the number of mole of H₂ required?
The number of mole of H₂ that are required to react with 3.2 moles Cl₂
can easily be calculated for by carefully observing the equation of the reaction. This is illustrated below:
The balanced equation for the reaction of hydrogen gas and chlorine gas is shown below:
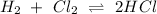
From the balanced equation show above, we can see that:
1 mole of Cl₂ reacted with 1 mole of H₂
Therefore,
3.2 moles of Cl₂ will definitely react with the same amount (i.e 3.2 moles) of H₂
Thus, we can conclude that the number of mole of H₂ required for the reaction is 3.2 moles