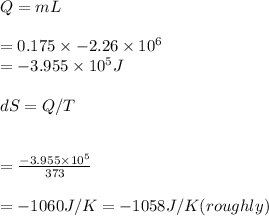Answer:
1- 214 J/K
2- -1058 J/K (roughly)
Step-by-step explanation:
1-Given:
mass of water
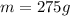
initial temperature
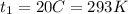
final temperature
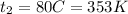
Specific heat capacity of water
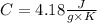
1-We can first calculate the change in entropy through the formula:
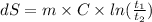
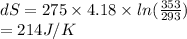
2-Given:
mass of water
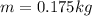
latent heat of vaporization
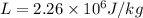
(In the case of condensation, we use a negative sign because the heat is expelled out of the system not inside of it)
heat emitted from the condensation of steam: