Answer:
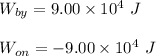
Option (c) is correct.
Step-by-step explanation:
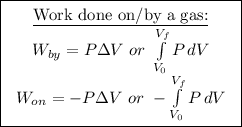
Given:
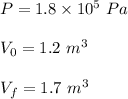
Find:
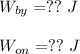
(1) - Calculating the change in volume
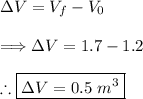
(2) - Calculating the work done by the gas
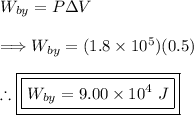
(3) - Calculating the work done on the gas
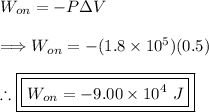
Options (a) and (d) can be eliminated. Option (b) can be eliminated since there is no negative in front of the answer. This leaves the correct answer being option (c).