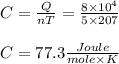Answer:
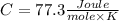
Step-by-step explanation:
Given:
Heat input:
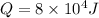
no. of moles:

change in temperature:

Since the gas is held at constant volume, we don't have to worry about any heat going into changing the volume.
We can find a formula to the specific heat capacity of the gas through the equation of heat:
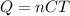
(The original equation is
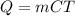 , but you since he asked for the unit to be Joule/mol x K, we can replace the mass with the number of mols)
, but you since he asked for the unit to be Joule/mol x K, we can replace the mass with the number of mols)
by substitution, we get: