Answer:
2.2241 Kg
Step-by-step explanation:
Given that it takes
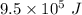 of energy to convert a block of ice at −15° to water at 15°. Find the mass of the block of ice. We'll call
of energy to convert a block of ice at −15° to water at 15°. Find the mass of the block of ice. We'll call
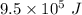 "
"
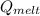 " which is the total energy taken to melt the block of ice.
" which is the total energy taken to melt the block of ice.
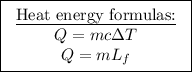
The energy it takes to raise the temperature of the ice from −15° to 0°:
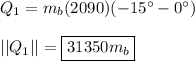
The energy it takes to convert the ice to water:
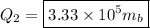
The energy it takes to raise the temperature of the water from 0° to 15°:

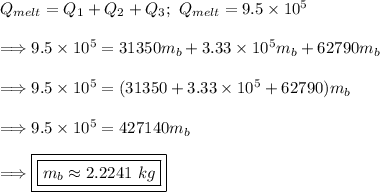
Thus, the mass is found.