Final answer:
The iron(III) thiocyanate concentrations are determined using stoichiometry for the stock solution and dilution calculations for the diluted solutions, reported with the correct number of significant figures.
Step-by-step explanation:
To calculate the concentration of the iron(III) thiocyanate complex ion for both the stock solution and the diluted solutions, we apply the concept of dilution and stoichiometry based on the provided volumes and concentrations.
Stock Solution Calculation
Using the provided volumes and concentrations of the reactants, the concentration of iron(III) thiocyanate in the stock solution is already given as 0.000200 M. This is calculated by stoichiometrically combining equal molar amounts of
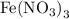 and KSCN, given that the concentration of iron(III) thiocyanate complex ion is directly related to the limiting reagent, which in exact stoichiometric proportions is neither in this case.
and KSCN, given that the concentration of iron(III) thiocyanate complex ion is directly related to the limiting reagent, which in exact stoichiometric proportions is neither in this case.
Diluted Solutions Calculation
For diluted solutions, we apply the dilution formula C1V1 = C2V2, where C1 is the concentration of the stock solution, V1 is the volume of the stock solution, C2 is the concentration of the diluted solution, and V2 is the total volume of the diluted solution.
Solution 1: 0.000200 M (unchanged from the stock solution as no dilution occurred)
Solution 2: C2 = (0.000200 M × 2.00 mL) / (2.00 mL + 2.00 mL) = 0.000100 M
Solution 3: C2 = (0.000200 M × 2.00 mL) / (2.00 mL + 4.00 mL) = 0.000067 M