Answer:

Step-by-step explanation:
Since we are given the specific heat, mass, and change in temperature, we should use this formula for heat energy.
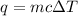
The mass is 2 kilograms. Iron's specific heat is 445 Joules per kilogram degree Celsius. The temperature increases by 150 degrees Celsius.
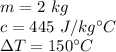
Substitute the values into the formula.
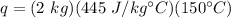
Multiply the first two numbers. The kilograms will cancel.
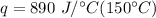
Multiply again. This time the degrees Celsius cancel.
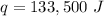
The pan absorbs 133,500 Joules of heat energy.