Final answer:
The standard free energy change (ΔG°) of the galvanic cell reaction can be calculated using the equation ΔG° = -nFE°, where n is the number of moles of electrons transferred, F is Faraday's constant, and E° is the standard cell potential.
Step-by-step explanation:
Calculating the Standard Free Energy Change (ΔG°)
The student has asked about the standard free energy (Δg°) of the reaction represented by the cell shorthand: Pt | Fe²+ , Fe³+ || Ag+ | Ag. To determine this, we must use the relationship between the standard free energy change, standard cell potential (ΔG° = -nFE°), and the number of moles of electrons transferred in the reaction (n).
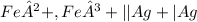
The Faraday's constant (F) is a known value used to convert between electrical energy and chemical energy.
First, we calculate the standard cell potential (E°) for the reaction using the standard potentials of the half-reactions. The half-reactions for this cell are Ag+ + e⁻ → Ag(s) with an E° of +0.80V and Fe³+ + e⁻ → Fe²+ with an E° of +0.77V. The E° cell is the difference between the cathode and anode potentials.
Next, we identify the number of moles of electrons transferred (n) in the balanced equation for the reaction. Lastly, we plug these values into the equation ΔG° = -nFE° to calculate the standard free energy change.
Spontaneity is indicated by a negative ΔG° and a positive E°.
The standard free energy change can also be calculated from standard free-energy of formation values (ΔG°f) of the reactants and products.