Solution :
Structure I
The formal charge on both Carbon (C) atom is = 4 valance
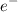 - bonds = 0
- bonds = 0
Formal charge (O) = 6 V.E - 2 bonds - 4 non bonding electrons = 0
Formal charge on (N) = 5 V.E - 3 bonds - 2 non bonding electrons = 0
F.C. on H = 1 V.E. - 1 bond = 0
Overall charge on the molecule = 0 charge
Structure II
Formal charge on both C atom = 4 valence
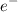 - 4 bonds = 0
- 4 bonds = 0
Formal charge (O) = 6 V.E. - 1 bonds - 6 non bonding electrons = -1 charge
Formal charge on (N) = 5 V.E. - 4bonds - 0 non bonding electrons = +1 charge
F.C on H = 1 V.E. - 1 bond = 0
Overall charge on the molecule = +1 -1
= 0 charge