Answer:
The volume of the gas is approximately 19.4 liters.
Step-by-step explanation:
We can use the Ideal Gas Law to solve for the volume of the gas:

Where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
First, we need to convert the temperature to Kelvin by adding 273.15:

Next, we can plug in the values and solve for V:
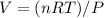
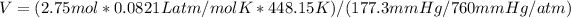
V ≈ 19.4 L