Answer:
aluminium bromide
Step-by-step explanation:
This is a synthesis reaction, where 2 individual molecules react to produce a single new compound.
The balanced chemical equation for the synthesis of aluminium metal and bromine gas is thus:
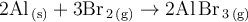
The product is therefore:
Aluminium bromide