The pH of a solution that results from mixing 25. 0 mL of 0. 200 M HA with 12. 5 mL of 0. 400 M NaOH. is 1.70.
To answer your question, we need to use the principles of acid-base chemistry and stoichiometry. HA represents a weak acid, and NaOH represents a strong base. When these two substances are mixed together, they will undergo a neutralization reaction. The balanced chemical equation for this reaction is: HA + NaOH →
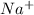 + H2O. Where NaA represents the salt that is formed from the acid and base.
+ H2O. Where NaA represents the salt that is formed from the acid and base.
To determine the pH of the resulting solution, we first need to calculate the moles of each reactant that are present in the mixture. We can use the formula: moles = concentration x volume (in liters)
For HA:
moles HA = 0.200 M x 0.0250 L = 0.00500 mol
For NaOH:
moles NaOH = 0.400 M x 0.0125 L = 0.00500 mol
Since the reaction between HA and NaOH is a 1:1 reaction, we can see that they react completely. Therefore, the number of moles of the excess reactant will be equal to the difference between the moles of the two reactants: moles excess = |moles HA - moles NaOH| = |0.00500 mol - 0.00500 mol| = 0 mol
Since there is no excess reactant, we can assume that all of the acid and base have reacted to form the salt
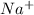 and water. The moles of the salt Na that are formed will be equal to the moles of the acid or base that are consumed in the reaction: moles
and water. The moles of the salt Na that are formed will be equal to the moles of the acid or base that are consumed in the reaction: moles
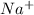 = moles HA (or moles NaOH) = 0.00500 mol
= moles HA (or moles NaOH) = 0.00500 mol
We can now use the volume of the mixture to calculate the concentration of the salt
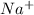 : concentration
: concentration
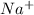 = moles
= moles
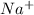 / volume (in liters) = 0.00500 mol / (0.0250 L + 0.0125 L) = 0.100 M
/ volume (in liters) = 0.00500 mol / (0.0250 L + 0.0125 L) = 0.100 M
Finally, we can use the principles of weak acid-base chemistry to determine the pH of the solution. Since
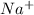 is the conjugate base of the weak acid HA, it will hydrolyze in water to produce OH- ions:
is the conjugate base of the weak acid HA, it will hydrolyze in water to produce OH- ions:
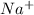 +
+
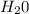 → HA +
→ HA +

The equilibrium constant for this reaction is given by:
Kb = [HA][
 ] / [
] / [
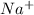 ]
]
We can assume that the concentration of
 ions is equal to the concentration of NaOH that was added to the mixture (since NaOH is a strong base and completely dissociates in water). Therefore: Kb = [HA][0.400 M] / [0.100 M] = 1.60 x 10^-13
ions is equal to the concentration of NaOH that was added to the mixture (since NaOH is a strong base and completely dissociates in water). Therefore: Kb = [HA][0.400 M] / [0.100 M] = 1.60 x 10^-13
The relationship between Ka (the acid dissociation constant) and Kb for a weak acid and its conjugate base is: Ka x Kb = Kw. Where Kw is the ion product constant for water (1.00 x 10^-14 at 25°C). Therefore: Ka = Kw / Kb = 6.25 x 10^-2
Since we know the value of Ka for HA, we can use the Henderson-Hasselbalch equation to calculate the pH of the solution: pH = pKa + log([A-]/[HA]). where pKa = -log(Ka) = 1.20 (for HA), and [A-]/[HA] = [
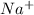 ]/[HA] = 0.100 M / 0.200 M = 0.500. Therefore: pH = 1.20 + log(0.500) = 1.70
]/[HA] = 0.100 M / 0.200 M = 0.500. Therefore: pH = 1.20 + log(0.500) = 1.70
In conclusion, the pH of the solution that results from mixing 25.0 mL of 0.200 M HA with 12.5 mL of 0.400 M NaOH is 1.70.