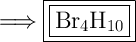Answer:

Step-by-step explanation:
This is an example of a molecular compound. A molecular compound consists of molecules whose formula represents the actual number of atoms bonded together in the molecule.
The prefixes in front of each molecule signify how many of each molecule there is in the total compound. Increasing from 1 onwards:
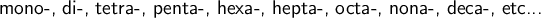
Therefore, for the above formula:
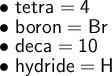
Hence: