The concentration of acid which is needed to completely neutralize 25.0 mL of 1.5 M NaOH, if it takes exactly 12.5 mL to reach the endpoint of the titration would be 3M.
We know that at the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
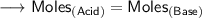
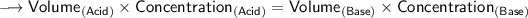
According to the specific parameters-
Now that we have all the required values,so we can plug them into the formula and solve for concentration of acid -
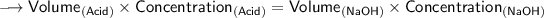
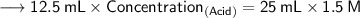
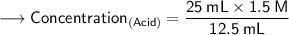
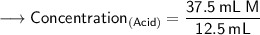



- Henceforth, the concentration would be 3M.