Answer: 6.65*10^-3
Step-by-step explanation:
The reaction below has the products and reactants reversed, so the Kp will be inversed (Kp^-1). The coefficients are also halved, so the Kp^-1 will be to the power of 1/2. This means that the Kp for the reaction below is
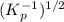 =
=
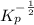 =
=
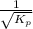 =
=
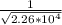 = 6.65*10^-3
= 6.65*10^-3