Answer:
150 mL
Step-by-step explanation:
Recall that

Now, we are given a Molarity of 0.250 M H2SO4 with a volume of 45 mL. We will convert this to moles
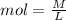
Then, since the mole ratio from H2SO4 to NAOH is 1:2, we will multiply by 2
Finally, we will get the volume by dividing by the Molarity of NaOH which is 0.150 M.
The setup for the answer is as follow
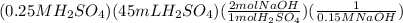
The final answer is 0.150 L or 150 mL.