Step-by-step explanation:
pH is defined as the negative logarithm of the hydrogen ion or hydroxonium ion concentration of a solution. That is;
![\bold{pH = -log([{H_3O}^(+)])}](https://img.qammunity.org/2024/formulas/chemistry/college/868nt6drdcyu52sxogqhx2vz8z0c004t6a.png)
From the question
![[{H_3O}^(+)]](https://img.qammunity.org/2024/formulas/chemistry/high-school/n57uuhsn5h8f3iy6jv1l0m48994wpz40jz.png) = 2.5 × 10-⁷ M
= 2.5 × 10-⁷ M
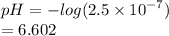
From the results the solution can be classified as acidic since it's pH is below 7 that's the neutral region